Compound 31d was found to be the most active compound with an IC 50 value of 0291 μM. The compound must have an acidic hydrogen on its β-carbon and a relatively poor leaving group on the α- carbonThe first step of an E1cB mechanism is the deprotonation of the β-carbon resulting in the formation of an anionic transition state such as a carbanion.
How To Identify The Electrophilic And The Nucleophilic Sites Of Amphetamine Quora
Formaldehyde f ə r ˈ m æ l d ə h aɪ d fər-MAL-də-hide also f ɔːr ˈ- for- systematic name methanal is a naturally occurring organic compound with the formula CH 2 O HCHO.
. The studies with SAR analysis also revealed that thiosemicarbazide group played a very vital role for tyrosinase inhibition acylamino moieties crucial for enhancing the tyrosinase inhibitory activity and the acylamide substituent at 4-position on the phenyl ring increased the. Compounds that exhibit a covalent mechanism of action are typically avoided in drug discovery programmes largely owing to safety concerns. Site selectivity can sometimes be achieved through appropriate directing groups or.
Direct CH functionalization can quickly increase useful structural and functional molecular complexity13. The pure compound is a pungent-smelling colorless gas that polymerises spontaneously into paraformaldehyde refer to section Forms below hence it is stored as an aqueous solution. The first is the relative reactivity of the compound compared with benzene itself.
The Birch Reduction is an interesting reaction that appears to go against the desire for molecular stability. Video 3 Birch Reduction Reaction and Mechanism. Sustained response was defined as 50 decrease in the mean pain intensity from baseline to weeks 2 to 12 and Complete Response as an average pain intensity score1 during weeks 2 to 12.
Experiments have shown that substituents on a benzene ring can influence reactivity in a profound manner. Use the orthometapara system to name simple disubstituted aromatic compounds. Organic Chemistry Study Materials Practice Problems Summary Sheet Guides Multiple-Choice Quizzes.
Identify the ortho meta and para positions in a monosubstituted benzene ring. Results with hepatocytes show that amoxicillin cimetidine ibuprofen mefenamic acid and. A final type of carbonyl compound of note is aromatic ketones.
Here Singh and colleagues present the pharmacological. Draw the structure of a simple disubstituted aromatic compound given. For example a hydroxy or methoxy substituent increases the rate of electrophilic substitution about ten thousand fold as illustrated by the case of anisole in the virtual demonstration above.
A carbon-carbon triple bond may be located at any unbranched site within a carbon chain or at the end of a chain in which case it is called terminalBecause of its linear configuration the bond angle of a sp-hybridized carbon is 180º a ten-membered carbon ring is the smallest that can accommodate this function without excessive strain. Our objective is to identify metabolic interactions between TCE and 14 widely used drugs in human suspended hepatocytes and characterize the strongest using microsomal assays. Changes in concentrations of TCE and its metabolites were measured by headspace GC-MS.
Addition Reactions of Alkynes. These can be formed from acyl chlorides via a Friedel-Crafts reaction using aluminium chloride as a catalyst. You will find it - Its all here.
Specific focus on how to recognize Oxidation and Reduction based on reactantsproduct how to identify reagents and of course oxidative cleavage comparisons. There are two main requirements to have a reaction proceed down an E1cB mechanistic pathway. In a Friedel-Crafts reaction a hydrogen atom on a benzene ring in an aromatic compound is substituted for an acyl group to form an aromatic ketone as shown in figure 5.
Logistic regression was used to identify predictors of response and Complete Response and subgroups of patients who respond best to the capsaicin patch.
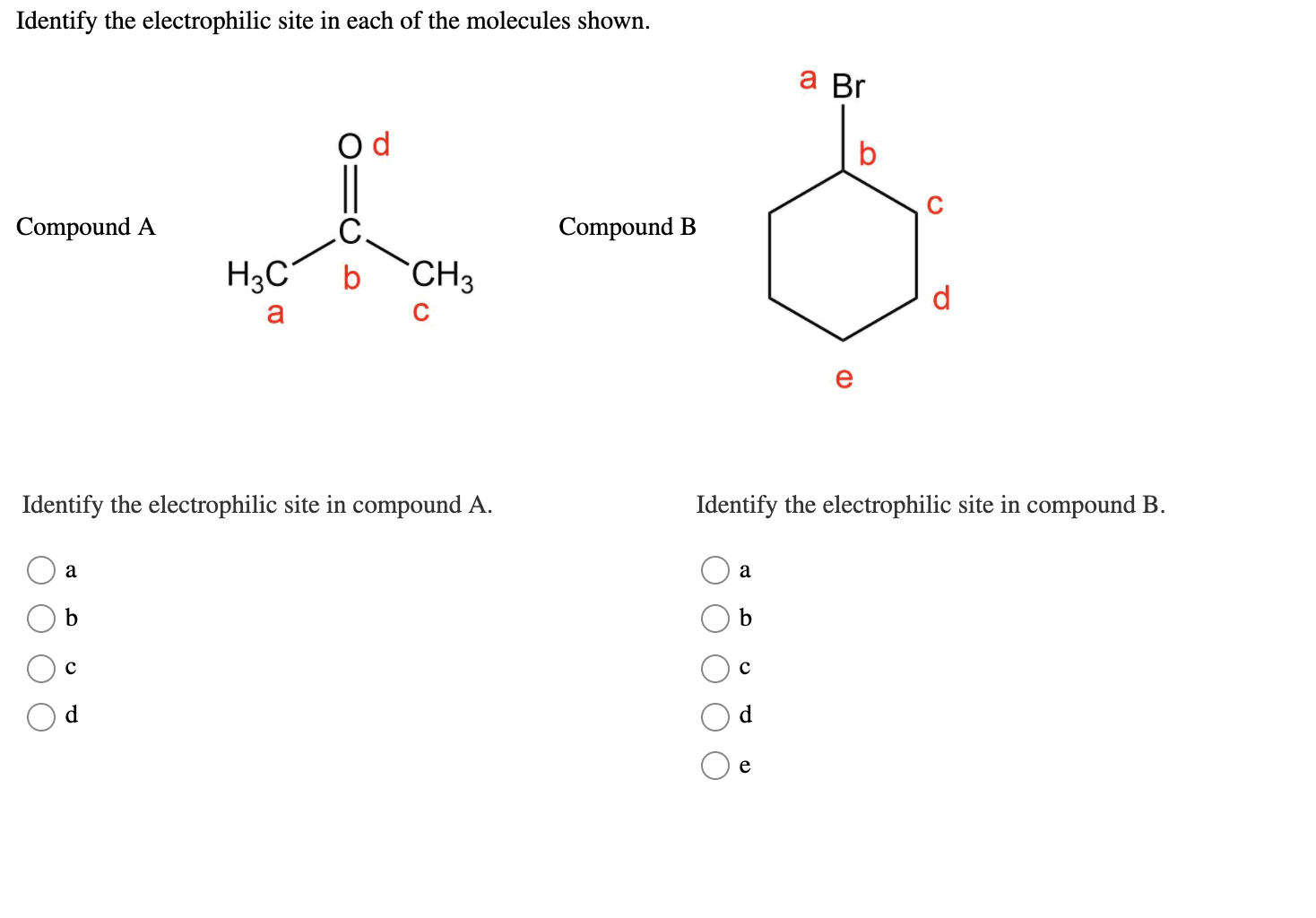
Solved Identify The Electrophilic Site In Each Of The Chegg Com

Solved Identify The Electrophilic Site In Each Of The Chegg Com

Identify The Electrophilic Site In The Following Molecule Study Com
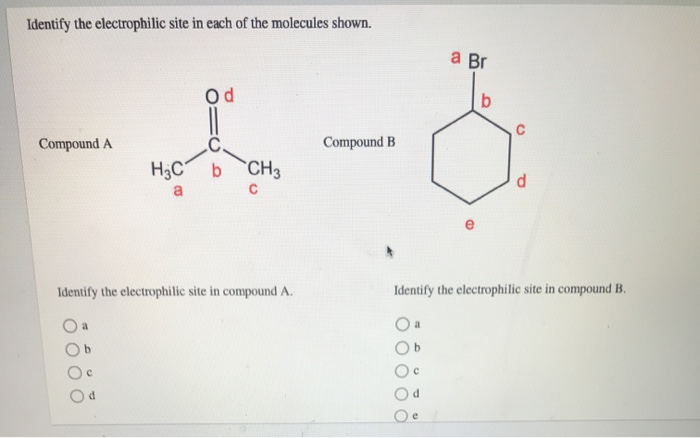
Solved Identify The Electrophilic Site In Each Of The Chegg Com
0 Comments